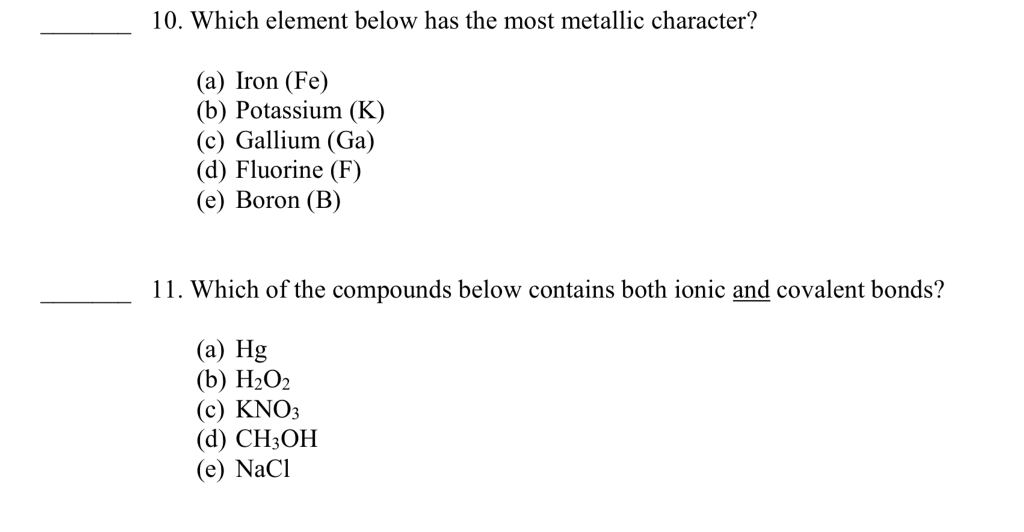
Which Element Is The Most Metallic . It exhibits some chemical properties similar to those of groups 1a and 7a. Learn chemistry +1 classification of elements and periodicity in properties.
Periodic Trends from xaktly.com
Some elements which shows metallic character are listed below. Among the elements b, al, c and si, (a) which element has the highest first ionization enthalpy (b) which element has the most metallic character?
Periodic Trends
It decreases from left to right and increases down a period in the periodic table. Sodium (furthest from fluorine) when a potassium atom reacts with a bromine atom, the potassium atom will. Learn chemistry +1 classification of elements and periodicity in properties.
Source: chemistrybytes.com
Recall that the trend for metallic character is as follows: Franium (element with highest metallic character) caesium (next highest level of metallic character) sodium; Which of the following group 2 (iia) elements has the most metallic character?
Source: 170188733453308075.weebly.com
Some elements which shows metallic character are listed below. From na, p, ca, br: Hence, rubidium is the most active amongst the given options.
Source: physicalsciencetext.weebly.com
It is not really a member of any particular group. It is the lightest element. In the given elements f, p, ba, and al, which one is the most electronegative?
Source: socratic.org
Answer the following question in relation to the above of element;. Sodium (furthest from fluorine) when a potassium atom reacts with a bromine atom, the potassium atom will. The elements with the most metallic character are on the left side of the periodic table (except hydrogen).
Source: xaktly.com
Element x is in group 2 (iia) and element y is in group 17 (viia). So, the correct answer is option d. List of elements atomic number name symbol group period number block state at.
Source: www.clutchprep.com
We’re being asked to determine the most metallic element among sodium, barium, magnesium, calcium, and cesium. It was considered a minor factor in the down fall of rome because citizens of rome (and greecused it’s acetate salt as a sweetening agent. From na, p, ca, br:
Source: www.slideserve.com
The alkaline earth element having the largest atomic radius is found in period 1, 2, 6, 7. So, thallium is most active metal among all. The natural element with the highest metallic character is cesium, which is found directly above francium on the periodic table.
Source: www.youtube.com
Which element is most reactive potassium aluminum or iron? A group of the element is the periodic table are given below. Which of the following atoms will lose an electron most readily?
Source: brainly.com
The element in period 3 with the most metallic character is. Among the elements b, al, c and si, (a) which element has the highest first ionization enthalpy (b) which element has the most metallic character? Some elements which shows metallic character are listed below.
Source: slideplayer.com
Which of the group 15 (va) elements can lose an electron most readily? Recall that the trend for metallic character is as follows: Among the elements b, al, c and si, (a) which element has the highest first ionization enthalpy (b) which element has the most metallic character?
Source: sciencenotes.org
The natural element with the highest metallic character is cesium, which is found directly above francium on the periodic table. The alkaline earth element having the largest atomic radius is found in period 1, 2, 6, 7. From na, p, ca, br:
Source: slideplayer.com
A group of the element is the periodic table are given below. So, thallium is most active metal among all. Click here👆to get an answer to your question ️ 31.
Source: www.chegg.com
(boron is the first member of the group and thallium is the last.) boron. 93 rows over 75% of the elements are metals, so they fill most of the periodic. So, the correct answer is option d.
Source: www.quora.com
It decreases from left to right and increases down a period in the periodic table. Which of the following atoms will lose an electron most readily? Its electron is not at all shielded from its nucleus.
Source: www.chegg.com
The natural element with the highest metallic character is cesium, which is found directly above francium on the periodic table. At room temperature which substance is the best conductor of heat. Which of the following atoms will lose an electron most readily?
Source: www.clutchprep.com
(boron is the first member of the group and thallium is the last.) boron. It is not really a member of any particular group. Sodium (furthest from fluorine) when a potassium atom reacts with a bromine atom, the potassium atom will.
Source: slidetodoc.com
The alkali metals in group 1 are the most active metals, and cesium is the last element in the group for which we have experimental data. Lose only 1 electrons (look at electron configuration) at room temperature, potassium is classified as a. In the given elements f, p, ba, and al, which one is the most electronegative?
Source: www.thoughtco.com
At room temperature which substance is the best conductor of heat. Learn chemistry +1 classification of elements and periodicity in properties. (a) 1s2 2s2 2p6 3s1 (b) 1s2 2s2 2p5 (c) 1s2 2s2 2p6 3s2 (d) 1s2 2s2 2p3.
Source: www.clutchprep.com
In the periodic table, reactivity decreases from left to right and increases from top to bottom. We’re being asked to determine the most metallic element among sodium, barium, magnesium, calcium, and cesium. From na, p, ca, br:
Source: slideplayer.com
Which of the group 15 (va) elements can lose an electron most readily? Which of the following group 2 (iia) elements has the most metallic character? The natural element with the highest metallic character is cesium, which is found directly above francium on the periodic table.