Which Of The Following Compounds Is An Aldehyde . Polyamino aldehydes and polyhalo aldehydes do not contain an oh group. Ch3ch2cho ch3ch2cooh ch3ch2ch2oh ch3ch2coch3 ch3ch2och3 question 5 which of the following compounds is an ester?
Solved Question 1 Which Of The Following Compounds Is An | Chegg.com from www.chegg.com
5)what is the common name for the following compound? The compound decanal contributes to the odor and flavor of an orange.
Solved Question 1 Which Of The Following Compounds Is An | Chegg.com
Go2 2) what are the bond angles in a typical carbonyl. (1)the simplest aldehyde is formaldehyde and the simplest ketone is acetone. 4) phenones are_____ a) aldehyde in which carbonyl group is attached with benzene ring.
Source: www.chegg.com
D) (c 6 h 5) 2 chch 2 coc 6 h 5. These carbonyl compounds consist of a central carbonyl carbon doubly bonded to an oxygen and single bonded to the r group (any alkyl group) and a hydrogen atom. These also do not produce oh substituted compounds on hydrolysis.
Source: www.chegg.com
Q the equation for the heat of combustion of each is the same: Name each of the following compounds by iupac rules. This test is not given by ketones.
Source: www.chegg.com
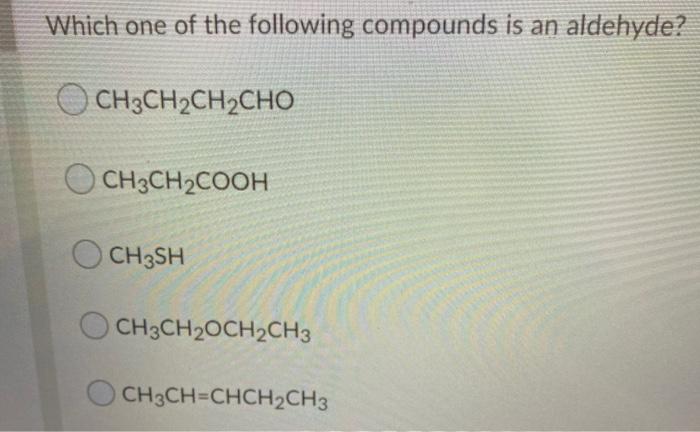
2)describe how the functional groups are similar in an aldehyde and a ketone. When sodium bisulphite is mixed with aldehyde it produces an additional white material which can be easily filtered to remove impurities. Which one of the following compounds is an aldehyde a.
Source: www.chegg.com
Acetone can strongly hydrogen bond to water. 100% (8 ratings) the correct answer is iv. These also do not produce oh substituted compounds on hydrolysis.
Source: slideplayer.com
2)describe how the functional groups are similar in an aldehyde and a ketone. B) carboxylic acids and esters. This test is not given by ketones.
Source: www.chegg.com
C4h8o + 11/2 o2 ® 4 co2 + 4 h2o. Classify each molecule as an aldehyde, ketone, or neither. An organic compound that has this frnctional group is 6.
Source: www.chegg.com
Acetone can strongly hydrogen bond to water. Which one of the following compounds is an aldehyde? The difference between ketone and aldehyde is the carbonyl group present in aldehydes can be easily oxidised to carboxylic acids whereas the carbonyl group in ketones are not oxidised easily.
Source:
Which of the following compounds is not named correctly? The compound decanal contributes to the odor and flavor of an orange. (1) in the iupac nomenclature system, an aldehyde group has priority over a ketone group.
Source: www.chegg.com
B) ketone in which carbonyl group is attached with benzene ring 3)draw an aldehyde containing only one carbon and name it. 4) phenones are_____ a) aldehyde in which carbonyl group is attached with benzene ring.
Source: slideplayer.com
C) 2° and 3° alcohols. Polyhydroxy carboxylic acids do not contain a cho or a keto group. D) (c 6 h 5) 2 chch 2 coc 6 h 5.
Source: study.com
The following tests are used to identify the presence of aldehydes and ketones. (3)reaction of a hemiacetal with an alcohol produces an acetal. Which one of the following compounds is an aldehyde?
Source: www.chegg.com
Where r stands for alkyl or aryl group. Aldehydes and ketones would always have a higher boiling point than the corresponding hydrocarbon with the same number of carbon atoms. B) carboxylic acids and esters.
Source: www.chegg.com
An organic compound in which a carbon atom is bonded to both a hydroxyl group. D) (c 6 h 5) 2 chch 2 coc 6 h 5. Which of the following compounds is not named correctly?
Source: www.bartleby.com
Which of the following compounds is not named correctly? (3)reaction of a hemiacetal with an alcohol produces an acetal. Drag the appropriate molecules to their respective bins.
Source: www.chegg.com
C) (c 6 h 5) 2 chch 2 cho. The following tests are used to identify the presence of aldehydes and ketones. The compound decanal contributes to the odor and flavor of an orange.
Source: www.numerade.com
In the given compounds, none of them has α − hydrogens. Acetals are prepared from ketones and alcohols. (4 points each) ch, b n.
Source: www.chemistryscl.com
Classify each molecule as an aldehyde, ketone, or neither. These also do not produce oh substituted compounds on hydrolysis. 4)which of the following compounds is a ketone?
Source: www.chegg.com
Which one of the following compounds is an aldehyde a. Which one of the following compounds is an aldehyde? A) alcohol c) ester ) aldehyde ether classified as a) an aci c) an ester b) an aldehyde d) a ketone o h c oh h c h c what is the iupac name for the compound that has the
Source: www.chegg.com
An organic compound in which a carbon atom is bonded to two alkoxy groups. Acetone can strongly hydrogen bond to water. These also do not produce oh substituted compounds on hydrolysis.
Source: www.chegg.com
Silver mirror test is used to distinguish between aldehydes and ketones. This test is not given by ketones. The following tests are used to identify the presence of aldehydes and ketones.