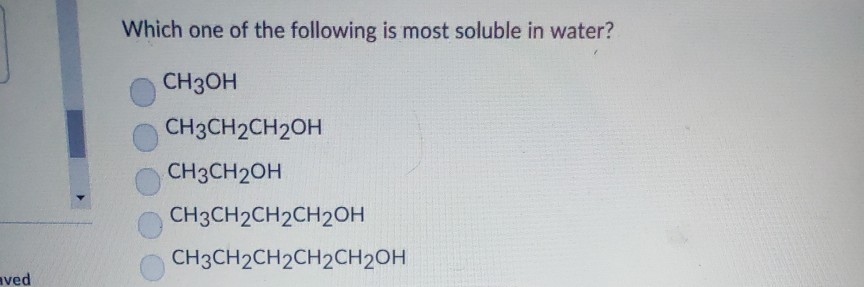
Which Of The Following Is Most Soluble In Water . Don't try to type the subscripts, just use letters and numbers. Greater is the number of hydrogen bonds, greater is the extent of hydrogen bonding and greater is the solubility in water.
Solved Which Molecule Is Most Soluble In Water? | Chegg.com from www.chegg.com
Calculate the solubility ‘s’ for each option, higher the value of ‘s’ higher the solubility. Rank the following substances in order from most soluble in water to least soluble in water:
Solved Which Molecule Is Most Soluble In Water? | Chegg.com
A complete resource book in chemistry. Enthalpy of a reaction can be measured b. Which of the following is the most soluble in water?
Source: www.bartleby.com
Which of the following phenomenon is con. Which of the following is more soluble in water, ethanol or ch3i (l). Some of the exceptions are c a s o 4, b a s o 4, p b s o 4, a g 2 s o 4 and s r s o 4.
Source: www.youtube.com
Hence, zns is the most soluble. The relation between solubility and solubility product constant is quite important when describing the solubility of slightly ionic compounds. Which of the following compound is most soluble in water?
Source: www.chegg.com
Which of the following is most soluble in water? A solution that contains 50 g of nh₄cl in 100 g of water at 50°c is _____ (saturated, unsaturated, supersaturated). To rank items as equivalent, overlap them.
Source: www.clutchprep.com
The most commonly used bleaching agent. Don't try to type the subscripts, just use letters and numbers. ( n o 3 −) are generally soluble.
Source: www.coursehero.com
Further there are few exceptions to this rule. (v) ch 3 ch 2 ch 2 ch 2 ch 2 ch 2 ch 2 ch 3. Calculate the solubility ‘s’ for each option, higher the value of ‘s’ higher the solubility.
Source: www.youtube.com
The law which states that the amount of. Which of the following is most soluble in water? Rank from most to least soluble in water.
Source: www.chegg.com
Greater is the number of hydrogen bonds, greater is the extent of hydrogen bonding and greater is the solubility in water. Formaldehyde, acetaldehyde, and acetone are soluble in water. Which compound is the most soluble in water?
Source: www.toppr.com
As the number of carbon atoms increases, the solubility of the compound in water decreases. Which of the following phenomenon is con. The relation between solubility and solubility product constant is quite important when describing the solubility of slightly ionic compounds.
Source: www.chegg.com
Which metal is more soluble in water? Hence, zns is the most soluble. The solubility of aldehydes is therefore about the same as that of alcohols and ethers.
Source: www.toppr.com
All sodium, potassium and ammonium salts are soluble in water. To rank items as equivalent, overlap them. Before attempting this question one must have prior knowledge about the solubility of alcohol in water, remember the factors due to which the solubility of alcohol increases in water, use this.
Source: www.youtube.com
Ch 3 ch 2 nh 2. A solution that contains 50 g of nh₄cl in 100 g of water at 50°c is _____ (saturated, unsaturated, supersaturated). To rank items as equivalent, overlap them.
Source: www.toppr.com
What of these is the chemical formula of. Table salt, or sodium chloride (nacl), the most common ionic compound, is soluble in water (360 g/l). Draw diagrams of ammonia, calcium iodide, and chloromethane (ch3cl) dissolved in water and identify the forces that allow for the.
Source: www.chegg.com
A solution that contains 50 g of nh₄cl in 100 g of water at 50°c is _____ (saturated, unsaturated, supersaturated). [ksp of agbr=5×10−13 and ksp of agcn s=1×10−12 ]. Calculate simultaneous solubility of agcn s and agbr in a solution of water.
Source: www.chegg.com
What of these is the chemical formula of. Hence, zns is the most soluble. Hgi2 is insoluble in water.
Source: www.chegg.com
As the number of carbon atoms increases, the solubility of the compound in water decreases. Among given compounds, ethylene glycol ( ho−ch2−ch2−oh ) is the most soluble in water. Sharing of 1 electron pair by one specie.
Source: www.youtube.com
Which of the following molecules is the most soluble in water? Ch 3 ch 2 ch 2 ch 2 nh 2. Enthalpy of a reaction can be measured b.
Source: www.chegg.com
The metallurgical process in which a met. To rank items as equivalent, overlap them. Most silver salts are insoluble.
Source: www.doubtnut.com
Table salt, or sodium chloride (nacl), the most common ionic compound, is soluble in water (360 g/l). Salts containing group i elements i.e. Greater is the number of hydrogen bonds, greater is the extent of hydrogen bonding and greater is the solubility in water.
Source: oneclass.com
Learn this topic by watching how to. Pbcl2, pbbr2, and pbi2 are soluble in hot water. As the number of carbon atoms increases, the solubility of the compound in water decreases.